Magnetic Exchange Force Microscopy with Atomic Resolution on the (001) Surface of the Antiferromagnetic Bulk Insulator Nickel Oxide
Using the short-ranged electron-mediated magnetic exchange interaction to map spin-structures with atomic resolution was predicted as early as 1990 [1]. However, the breakthrough experiment on the antiferromagnetic insulator NiO(001) was not successfully performed until 2006 and published in Nature in 2007 [2]. It should be noted that spin mapping with atomic resolution was demonstrated by our group with SP-STM already in 2000 [3]. However, this method is only applicable to conductive surface. Atomic scale magnetism of electrical insulating samples like, e.g., NiO(001), can only be probed with a force microscopy based technique, i.e., magnetic exchange force microscopy (MExFM). Note further that this MExFM is very different form MFM, which detects the long-range dipolar magnetic interaction between tip and sample [4]. The stray field above a surface stemming from many parallel aligned atomic spins in a ferromagnetic domain can be easily detected with this method. However, between two atoms this interaction is so weak that it cannot be detection with current force microscopy set-ups. Furthermore, antiferromagnetic samples cannot be studied at all, because their do not possess a stray field at all.
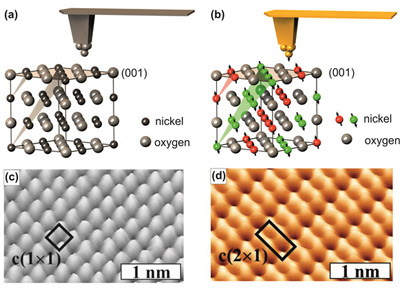
Conceptually, MExFM is very simple and presented in Fig. 1. Atomic force microscopy (AFM) is able to image insulators like NiO(001) with atomic resolution [5,6], if the tip apex ends in a single atom, i.e., if the tip is atomically sharp. Since NiO crystallizes in the NaCl structure Ni and O atoms are arranged in a checkerboard pattern on the (001) surface. The short-ranged electron mediated chemical interaction between foremost tip apex atom and the O and Ni surface atoms, respectively, is different, which is responsible for the site-dependent contrast formation. This situation is depicted in Fig. 1(a). Corresponding experimental data are displayed in (c). If the tip is magnetic, atomically sharp and brought close to the surface, the magnetic exchange interaction will show up as well, because it is electron mediated as well and therefore has approximately the same range as the chemical interaction. In NiO superexchange between Ni d-electrons via the bridging O atoms is responsible for the antiferromagnetic magnetic ordering. On the (001) surface a row-wise antiferromagnetic contrast on neighboring Ni atom along the directions emerges as depicted in Fig. 1(b). The resulting image in (d) shows the chemical contrast plus the additional magnetic contrast on the spin carrying Ni atoms.
The (1x1) chemical unit cell and the (2x1) antiferromagnetic unit cell are indicated in Fig.1 (c) an (d), respectively. Note that for metallic tips the maxima in AFM topography images on ionic surfaces like NiO(001) can be identified as the cations, i.e., O on NiO(001) [7].
A priori it is not entirely clear that the magnetic contrast is visible only on the spin carrying Ni atoms. In NiO superexchange via the bridging O atoms is responsible for the magnetic ordering. Thus, such a superexchange could also lead to a contrast on surface O atom stemming from the Ni atoms in the layer below. Furthermore, the broken symmetry at the surface results in a slight magnetic polarization of the O atoms at the surface. However, theoretical calculations [8] predict that the magnetic exchange interaction on the O atoms is present, but much smaller than on the Ni atom. Experimentally, we indeed observed a row-wise contrast on O atoms as well as on Ni atoms with MExFM. However, we interpret this contrast stems from a magnetic double tip that generates an apparent magnetic contrast on the O atoms [9].
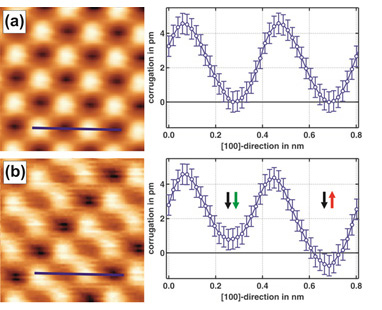
Figure 2 shows the distance dependence of the chemical interaction compared to the magnetic exchange interaction on NiO(001). Both images were obtained with the same tip, but (b) was recorded at a slightly smaller tip-sample separation than (a). Peculiarly, only (b) exhibits a magnetic contrast. NiO is an insulator, because strong correlations, i.e., on-site repulsion of the spin carrying d-electrons, prevent the formation of a conduction band. Hence, the Ni d-electrons are localized close to the nucleus and do not protrude far into the vacuum region. Therefore, at relatively large separations the tip only probes the p- and s-electrons, but not the d-electrons. Chemical contrast formation between Ni and O is possible, but no spin contrast between Ni atoms can observed. Only at sufficiently small separation, when the d-electrons are directly probed, the magnetic contrast becomes detectable. This phenomenon also explains why achieving magnetic resolution on NiO(001) is quite difficult: One has to approach very close to the surface, where large chemical interaction can lead to undesired tip modifications that result in non-magnetic or blunt tip apices. Moreover, it explains why the magnetic contrast on the antiferromagnetic Fe monolayer on W(001) [10] is much larger (4 to 5 times) than on NiO(001) and easier obtained: On such an itinerant metallic system the d-electrons are delocalized and thus protrude relatively for into the vacuum region.